 🖨️ Print post
🖨️ Print post
Chickenpox was once an almost universal childhood experience and a routine rite of passage. Nearly every child under the age of fifteen contracted the generally mild illness, experienced a full recovery after five to ten days and gained natural immunity for life. Today, however, chickenpox is much less common in the U.S. due to a mass varicella (chickenpox) vaccination program that reaches most children.
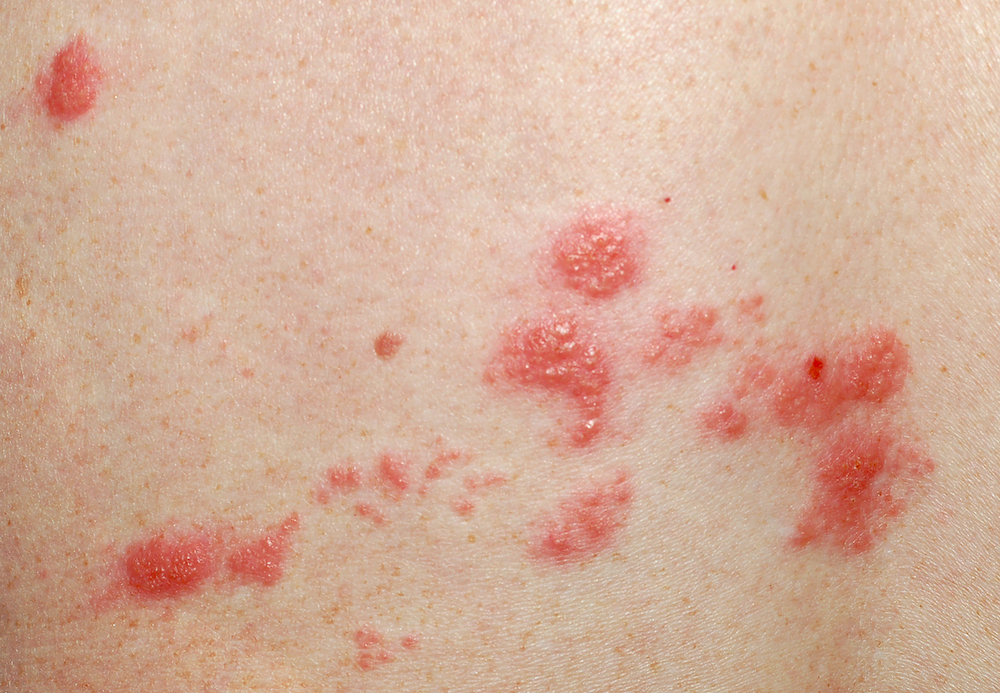
Chickenpox is a contagious disease caused by infection with the varicella-zoster virus—a member of the herpes virus family and the same virus that causes shingles. Chickenpox spreads through coughing and sneezing or by touching or breathing in the virus particles that come from chickenpox blisters. Prior to the advent of the varicella vaccine in 1995, chickenpox affected an estimated four million Americans annually (although surveillance data were spotty as only about half of all states reported any cases at all).1 Children who experienced wild chickenpox developed a distinctive blister-like rash, with blisters appearing first on the chest, back and face and then spreading over the whole body. Other potential symptoms included low-grade fever, headache, runny nose, fatigue, loss of appetite or itchiness. In most cases, chickenpox resolved on its own.
In 2000, researchers at the Centers for Disease Control and Prevention (CDC) estimated that the illness was responsible for an average of ninety deaths per year in the twenty-five years leading up to the vaccine’s introduction (1970- 1994).2 However, nearly three in ten deaths (28 percent) were in persons with preexisting high-risk conditions.2 Adults over fifty years of age reportedly accounted for another 19 percent of deaths, but different CDC authors later observed that “an unknown but large proportion of deaths attributed to varicella among individuals aged 50 years and older are likely to be herpes zoster or causes other than varicella.”3
Because chickenpox is generally not harmful in childhood, doctors used to commonly recommend intentionally exposing children to the disease. Parents held chickenpox parties, believing it was prudent to let their children get chickenpox at a young age so they would avoid the potentially more serious complications of contracting the disease as a teenager or adult. Nowadays, many parents choose to vaccinate their children to prevent chickenpox, and others are forced to vaccinate so that their children can attend public and private schools and daycare centers. Varicella is on the list of mandatory vaccines in all fifty states.4
VACCINE ADVERSE EVENTS
Two varicella vaccines are available in the U.S., both of which are live-virus vaccines made by the pharmaceutical company Merck. The U.S. Food and Drug Administration (FDA) approved Varivax in 1995 for use in people one year of age and older; Varivax is meant solely to prevent chickenpox. In 2005, the FDA also approved Merck’s ProQuad vaccine for use in children one through twelve years of age. ProQuad (MMRV) combines the varicella vaccine with the measles, mumps and rubella (MMR) vaccine. The CDC recommends two doses of varicella vaccine, with the first administered at twelve to fifteen months of age and the second administered between four and six years of age.
While avoiding chickenpox may sound like a good idea to some, there are several things one should consider prior to vaccinating, including the safety of the varicella vaccines. According to the CDC’s website, rare side effects caused by the vaccines include severe rash, infections of the lungs or liver, meningitis, seizures, viral and bacterial pneumonia and severe infection with the chickenpox virus from the vaccine.5 Data submitted to the federal government’s Vaccine Adverse Event Reporting System (VAERS)
confirm additional serious vaccine reactions that include cellulitis, cerebellar ataxia, convulsions, encephalitis, Guillain-Barré syndrome, necrotizing fasciitis, osteomyelitis, seizures, septic arthritis, septicemia, shingles, thrombocytopenia, toxic shock syndrome, transverse myelitis and death.
In the first three years of the varicella vaccine’s use—between 1995 and 1998—VAERS received more than sixty-seven adverse event reports per one hundred thousand doses of vaccine administered (or about one in fifteen hundred). Approximately 4 percent of those reports described serious adverse reactions. However, the true number was likely closer to one hundred adverse events per fifteen hundred doses. This is because a report commissioned by the U.S. Department of Health and Human Services concluded that fewer than 1 percent of all vaccine adverse events are ever reported to VAERS.6
QUESTIONABLE INGREDIENTS
Ingredients included in varicella-only vaccines include a weakened form of the varicella virus; bovine calf serum or fetal bovine serum; hydrolyzed gelatin; monosodium L-glutamate (MSG); and human MRC-5 cells (including DNA and protein), among other ingredients (see sidebar).7 The combination MMR-plus-varicella formulations also include genetically-engineered human albumin and sorbitol (a highly processed sweetener often made from corn and associated with digestive distress).7
The FDA claims that all of these ingredients are safe when used in vaccine production, but one could easily argue the contrary. For example, scientific studies conducted in Japan in the early 1990s identified anaphylactic reactions associated with gelatin in vaccines,8 but vaccine manufacturers continue to use gelatin as a stabilizer in eleven vaccines licensed and distributed in the U.S. Additionally, studies by Massachusetts Institute of Technology scientist Stephanie Seneff have shown that all commercial gelatin used in the U.S. is contaminated with the herbicide glyphosate (Roundup) as a result of current animal feeding practices.9
Manufacturers use MSG as both a preservative and stabilizer to keep varicella vaccines effective in response to heat, light, acidity and humidity. Most of us know of MSG as a flavor enhancer added to Chinese food, canned vegetables, soups and processed meats, and some of us may have experienced the flushing, headaches, muscle tightness, numbness, tingling and weakness that MSG can provoke. With long-term exposure, other common MSG symptoms include asthma, neurodevelopmental delays and seizures. The Mayo Clinic reports that the substance can cause disorientation, fatigue and heart palpitations.10 As an “excitotoxin,” MSG can mediate the death of central neuron receptors in the brain.11 Despite these findings, the FDA classifies MSG as “generally recognized as safe” (GRAS).
Bovine calf serum (called BSA) and fetal bovine serum are cow-derived products used to grow viruses in live vaccine production. BSA has been found to cause allergic reactions in humans.12 In addition, a study published in 2011 in The New England Journal of Medicine described the plausible association of BSA and a very difficult-to-treat form of kidney disease called idiopathic membranous nephropathy.13
MRC-5 is a diploid human cell culture line composed of fibroblasts derived from lung tissue of a healthy fourteen-week-old aborted Caucasian male fetus. Varicella vaccines do not contain the original aborted cells, but they do contain traces of human DNA, as do the MMR II vaccines that Merck combines with its varicella vaccine. The FDA has acknowledged that residual human DNA has the potential to cause cancer or to change one’s genetic code, so it is hardly reassuring that all vaccine package inserts include a section stating that the vaccines have never been evaluated for carcinogenic or mutagenic effects.14 A comprehensive study by Dr. Helen Ratajczak, a former senior scientist at a pharmaceutical company, found that spikes in the incidence of autism in the late 1980s and again in the mid-1990s coincided with the increased use of the human-DNA-contaminated MMR II and varicella vaccines.15
LESS PROTECTION
When varicella vaccine proponents make their case for vaccination and the suppression of wild chickenpox, chances are they are not disclosing the benefits of actually getting chickenpox. One of those benefits is protection against heart disease, including heart attacks and angina pectoris. In 2007, a study titled “Dual role of infections as risk factors for coronary heart disease,” published in the journal Atherosclerosis, concluded that contagious childhood diseases not only had a protective effect against acute coronary events but that each additional contagious disease contracted during childhood—such as chickenpox, measles, mumps or rubella—increased the protective effect by 14 percent.16
Another study—“History of chickenpox in glioma risk: a report from the Glioma International Case-Control Study (GICC),” published in 2016 in Cancer Medicine—found that a history of chickenpox infection was associated with a 21 percent lower probability of later experiencing glioma, a deadly brain cancer.17 The protective effect was even stronger for high-grade brain cancers.
DIMINISHING RETURNS
Over time, universal chickenpox vaccination has become less effective, prompting booster doses that are not as protective as the immunologic boosting that occurred naturally in the pre-vaccine era.18 A 2017 study of Air Force recruits (comparing varicella vaccination and natural infection) found that vaccine-induced immunity decreased by 8 percent with each year post-vaccination—and varicella-vaccinated young adults were at increased risk for varicella outbreaks compared to those who had experienced ordinary chickenpox infection.19
Individuals can also contract chickenpox due to vaccine failure, known as “breakthrough” varicella disease. Breakthrough illness is considered to occur when a vaccinated person contracts wild-type varicella virus more than forty-two days after vaccination. FDA researchers report that approximately one in ten vaccinated children develop breakthrough disease following exposure to chickenpox.20
According to the National Vaccine Information Center (NVIC), there have been multiple outbreaks of chickenpox among fully vaccinated schoolchildren, and vaccinated students are frequently found to be responsible for the outbreaks.21 Describing the period from 2001 to 2005, NVIC notes that at the same time that the CDC was claiming vaccine effectiveness of 72 to 85 percent, some schools were reporting chickenpox in up to 40 percent of vaccinated students in a single classroom.21 In response to the significant rise in illness among vaccinated children, the CDC’s Advisory Committee on Immunization Practices (ACIP) recommended in 2006 that all children get a second dose of chickenpox vaccine prior to school entry.
Previously vaccinated individuals often report their illness as a milder infection, with fewer lesions and a rash that presents as papules (raised itchy bumps) instead of vesicles (fluid-filled blisters). However, during the documented outbreaks in the early to mid-2000s, up to 30 percent of cases were not mild.21 In those cases, vaccinated students experienced chickenpox symptoms similar to children who developed natural chickenpox.
Recently vaccinated individuals can spread the varicella virus to others through a process called viral shedding—a long-known vaccine complication reported in the medical literature.22 This can happen when an uninfected person comes in close contact with a recently vaccinated person’s body fluids. Although public health officials claim shedding is “rare,” package inserts for chickenpox vaccines openly state that “transmission of varicella vaccine virus may occur” and warn that vaccinated individuals should avoid close contact with infants, pregnant women and immunocompromised individuals for up to six weeks.23
SHINGLES: A PAINFUL TRADEOFF
Chickenpox vaccines are expensive, costing between one hundred twenty dollars and two hundred twenty dollars per dose. However, universal chickenpox vaccination may be exacting an even higher price in the form of an iatrogenic epidemic of shingles (herpes zoster). There is strong evidence that the incidence of shingles is increasing precisely because the chickenpox vaccine is preventing people from receiving the natural boosting effects of regularly circulating wild-type chickenpox.
Studies show that before routine use of the chickenpox vaccine, individuals who recovered from chickenpox as children later experienced recurrent asymptomatic boosting of immunity by coming in contact with others infected with chickenpox. Parents and grandparents who interacted with their infected children and grandchildren were, therefore, less likely to develop shingles because they had an opportunity to reinforce their immunity. Without this periodic asymptomatic boosting, the dormant varicella-zoster virus can reemerge as shingles, especially in the elderly and in those with a weakened immune system.24
Shingles is a painful disease that causes the eruption of a blistery rash, usually on one side of the face or body. Before the rash appears, the affected person may have nerve symptoms of pain, itching, burning or tingling. The rash itself may be uncomfortable, but it is the internal irritation from the affected nerve root that causes many individuals to experience the more intense pain associated with shingles. Although shingles is not contagious, people with shingles can spread chickenpox to those who have neither had varicella disease nor the vaccine.
The shingles rash usually clears up after two to four weeks, yet some individuals experience pain that can last for months or even years. This condition, called post-herpetic neuralgia (PHN), generally affects one in ten people who get shingles. Other complications can occur when shingles infects the eye, which can damage the cornea and cause problems with vision, including blindness. Rarely, shingles can also lead to pneumonia, hearing problems, brain inflammation or death.25 Shingles results in approximately ninety-six deaths per year, similar to the number of deaths caused by chickenpox before the introduction of the varicella vaccine.26
The CDC currently estimates that one in three people will develop shingles during their lifetime,26 but before routine use of chickenpox vaccines, the agency’s estimate was that just 15 percent of adults (approximately one in seven) would experience shingles at some point.1 The CDC maintains that the condition usually occurs in adults over the age of fifty—and that the risks of getting the disease and having serious complications increase with age—but a growing number of studies show more diagnosis of shingles in children since the CDC added the chickenpox vaccine to the childhood vaccine schedule. Prior to the advent of chickenpox vaccination, clinicians did not expect children to get shingles unless they were seriously immunocompromised.
Regrettably, children are developing shingles from the chickenpox vaccine itself—as proven by molecular analysis. A 2009 study titled “The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination,” published in The Pediatric Infectious Disease Journal, reported that the incidence of shingles jumped by 63 percent among young people ten to nineteen years of age in the U.S. from 2000 to 2006.27 Reluctant to blame chickenpox vaccination, the researchers stated only that this increase “could not be confidently explained” and continued to advocate the vaccine’s “widespread use.”27 North Carolina researchers who reviewed roughly two dozen published cases of vaccine-strain shingles in children and adolescents found that the average age of infection was 5.3 years, with infection occurring, on average, about three years after chickenpox vaccination.28
In 2011, researchers examined laboratory characteristics of fourteen suspected shingles cases in varicella-vaccinated children—including three children who had experienced natural chickenpox in infancy before receiving their vaccines; the researchers concluded that a third of the confirmed shingles cases “were due to vaccine-type virus.”30
The incidence of shingles is also on the rise in the adult population. A 2005 Massachusetts study that examined chickenpox and shingles incidence “during a period of increasing varicella vaccine coverage” (1999-2003) determined that shingles incidence increased by 90 percent across all age groups—but it rose by a whopping 161 percent in persons aged twenty-five to forty-four.29
NEW PROBLEMS=NEW VACCINES
In 2006, in response to the burgeoning shingles epidemic, Merck (the same manufacturer that produces the chickenpox and MMR II vaccines) launched the first shingles vaccine, Zostavax. Like the chickenpox vaccine, Zostavax is a live-attenuated vaccine, meaning it too contains a weakened form of the varicella-zoster virus. The vaccine’s other ingredients are similar to those in the company’s varicella vaccines (see sidebar).7 Because Zostavax is a live-attenuated vaccine, it also has the potential to cause the disease it is meant to prevent. The CDC admits as much, stating that, rarely, “live shingles vaccines can cause rash or shingles.”31 In 2014, the FDA required that Merck add a warning about the potential for vaccine-strain infection to the list of side effects associated with its shingles vaccine.32
From the outset, Zostavax has been plagued by questions about its safety. A 2015 study in the Journal of Drugs in Dermatology described a significantly increased risk of developing two autoimmune conditions—alopecia (hair loss) and arthritis—in those who had received the shingles vaccine.33 Although vaccinated seniors were more than twice as likely to subsequently develop the two conditions, the authors dismissed the adverse outcomes’ importance as being non-life-threatening and pronounced the vaccine “relatively safe.”33
Other researchers disagree, however. In a 2013 letter published in the New England Journal of Medicine, a Bethesda, Maryland, geriatric expert cited a statistically significant 36 percent increase in the rate of serious adverse events associated with shingles vaccination in persons sixty years of age or older and pronounced the vaccine’s safety “questionable.”34 Despite these concerns, Merck distributed in excess of thirty-six million doses of Zostavax from 2006 to 2017, earning an average of six hundred eighty-five million dollars annually—and seven hundred forty-nine million dollars in 2017 alone.35
In his New England Journal of Medicine letter, the geriatric expert also observed that the shingles vaccine’s efficacy has been vastly overstated.34 In fact, as NVIC summarizes, efficacy studies have found a significant decrease in the vaccine’s effectiveness just one year post-vaccination, “and by nine years, Zostavax was determined to be no longer effective at preventing shingles.”36 Its own package insert states, “The duration of protection beyond 4 years after vaccination with Zostavax is unknown.”37
Merck has faced (and continues to face) a multitude of lawsuits pertaining to Zostavax, with autoimmune disorders, cardiovascular issues, congestive heart failure, hearing loss, brain inflammation, necrotizing retinitis, spinal cord inflammation, stroke, vasculitis, death and shingles all associated with the vaccine.38 Claimants allege that Merck produced and sold “an unreasonably dangerous vaccine” and assert that the company knew—or should have known—that the vaccine was not safe.38 To make matters worse, these injured individuals may have suffered in vain; a physician at the University of California-Los Angeles (UCLA) has pointed out that one hundred and seventy-five people would need to receive the Zostavax vaccine to prevent one case of shingles.39 (Note that adults injured by shingles vaccines are fortunate to be able to sue manufacturers for compensation—if the vaccine had been designed for children and recommended by the CDC, the spurious National Childhood Vaccine Injury Act of 1986 would protect manufacturers from any and all liability.)
MORE PROBLEMS=MORE NEW VACCINES
Unfortunately for Merck, a second shingles vaccine called Shingrix became available in 2017, manufactured by GlaxoSmithKline (GSK). The CDC currently recommends Shingrix as the preferred shingles vaccine because of its greater reported effectiveness. Shingrix claims to be up to 90 percent effective at preventing shingles, compared to Zostavax’s official estimate of 51 percent.40
GSK is aggressively marketing two doses of Shingrix to adults over the age of fifty. Since its 2017 approval, the vaccine has been a top growth engine for the company, reaching sales of 1.6 billion dollars in the first nine months of 2019.45 The retail cost of the vaccine is around two hundred eighty-two dollars for the two injections, compared to about two hundred twenty dollars for the single-dose Zostavax vaccine.
As with Zostavax, a significant number of common and severe side effects have been reported following the administration of Shingrix. Within the first four months of Shingrix being on the market, VAERS had received one hundred fifty-five adverse event reports linked to the vaccine.46 According to Dr. Kathleen Dooling of the CDC’s Division of Viral Diseases, more than 70 percent of clinical trial participants experienced pain after getting the Shingrix vaccine, and “about one in six people experienced side effects so severe that it actually prevented their normal activities.”40 The Shingrix package insert lists adverse reactions that include allergic reactions (such as rash, hives and swelling of the face, tongue or throat capable of causing difficulty in swallowing or breathing), chills, fever, generally feeling unwell, headache, injection site itching, muscle pain, redness and swelling at the injection site.47
MANY NEGATIVES, NO PLUSES
Merck and GSK—with help from the CDC—both claim that even if their herpes zoster vaccines fail to protect recipients from a bout with shingles, the vaccines will make the rashes less painful and help clear them up more quickly. Is this uncertain benefit worth it? By May 2019, the number of vaccine reactions, hospitalizations, injuries and deaths reported to VAERS following vaccination with either Zostavax or Shingrix had climbed to over sixty-one thousand, including one hundred seventy-nine deaths, over two thousand hospitalizations and over one thousand related disabilities.48
With all of these negatives, it is hard to imagine why officials continue to recommend chickenpox and shingles vaccines so fervently. The answer seems to lie in some combination of underlying profit motives and irrational fear, both of which have clouded the judgment of vaccine manufacturers, public health officials and lawmakers. Among the numerous unfortunate consequences of these misguided policies are an upward shift in the age distribution of chickenpox cases from children (who generally recover with no complications) to teenagers and adults (who have higher complication rates), as well as a painful shingles epidemic that has shifted downward the age at which someone gets shingles. Ironically, the FDA predicted a potentially significant rise in shingles cases prior to the chickenpox vaccine’s introduction in 1996 but said nothing when the CDC ignored its recommendation to monitor shingles rates closely.21
While some hail childhood vaccines—including the chickenpox vaccine—as a victory over disease, others argue that the multitude of vaccines given to children are causing huge increases in chronic immune and brain dysfunction. Currently, the CDC’s recommended childhood schedule includes sixty-nine doses of sixteen different vaccines. Meanwhile, almost one in three children has an allergy,49 one in six has a developmental disability,50 one in nine has ADHD,49 one in twelve has asthma,51 one in thirteen has severe food allergies49 and one in thirty-six has autism.52
Those who promote vaccination also make the assumption that all infectious diseases are bad—but are they really? Doctors have long known that a bout with minor illness strengthens the immune system. This has not prevented public health officials and the media from expounding on the “dangers” of chickenpox, an illness that was typically so benign that it did not even become a nationally notifiable illness until 1972.
Of course, the bottom line is that the decision to vaccinate should never be in the hands of doctors, educational institutions or the government. It is the responsibility of every parent and individual to weigh the risk of the disease against the risk of vaccination side effects. We must remember that vaccination choice is a fundamental human right, and only with truly informed consent for all medical interventions can we ensure that we live in a free society. Our bodies belong to us—and us only.
SIDEBARS
WHAT’S IN THAT VACCINE—AND WHY IS IT THERE?
In addition to the antigen (the weakened form of the virus), Merck’s varicella-only and herpes zoster vaccines include ingredients such as the following:
• Bovine calf serum or fetal bovine serum: component of growth medium
• Ethylenediamine-tetraacetic acid (EDTA): preservative (also a chelating agent)
• Hydrolyzed gelatin (bovine or porcine): stabilizer
• Monosodium L-glutamate (MSG): stabilizer
• Neomycin: antibacterial
• Potassium chloride: medium nutrient, adjusts pH and tonicity
• Sodium chloride: adjusts tonicity
• Potassium phosphate monobasic, sodium phosphate monobasic and/or sodium phosphate dibasic: adjusts pH
• Sucrose: stabilizer
• Human MRC-5 cells (including DNA and protein): “manufacturing residue”
• Urea: stabilizer
Merck’s combination MMRV vaccine includes similar ingredients plus recombinant (genetically-engineered) human albumin (used as a component of the growth medium) and sorbitol (used as a stabilizer and solvent).
The ingredients of GlaxoSmithKline’s Shingrix vaccine include a genetically-engineered form of the virus as well as many of the same ingredients as Merck’s vaccines. In addition, Shingrix contains:
• QS-21 and MPL: adjuvant suspension
• Dioleoyl phosphatidylcholine (DOPC): adjuvant
• Cholesterol
• Polysorbate 80: surfactant
• Host cell protein and DNA
QUESTIONABLE INGREDIENTS IN GLAXOSMITHKLINE’S SHINGLES VACCINE
Shingrix is an inactivated, genetically-engineered vaccine that does not contain a live virus like its competitor Zostavax. The vaccine’s primary ingredients include glycoprotein E (gE)—a protein found in the varicella-zoster virus—mixed with GSK’s proprietary adjuvant suspension called AS01.41 The latter consists of the saponin QS-21—a purified extract from the bark of a soapbark tree native to central Chile (Quillaja saponaria Molina)—and an immune-stimulating fat called MPL (3-O-desacy1-4’-monophosphoryl lipid A). The highly potent QS-21 adjuvant is used to stimulate a strong immune response in lieu of using a live virus, but it is experimental and little is known about its mechanisms of cellular activation.42 Some researchers have reported uncertainty about QS-21’s potential toxicity and “undesirable haemolytic effect” (rupturing of red blood cells and release of their contents into surrounding fluid) in humans.43 Shingrix also contains the problematic surfactant polysorbate 80, associated with adverse effects on female reproduction and blood-brain barrier permeability.44
HOME CARE FOR CHICKENPOX
Home remedies that may help relieve chickenpox symptoms and prevent skin infections include:
• Taking baths with baking soda or oatmeal
• Applying cool compresses
• Applying raw honey, diluted apple cider vinegar or a baking soda paste
• Using a homemade calamine lotion with bentonite clay, baking soda, sea salt and soothing essential oils
• Taking natural immune-boosters such as astralagus, calendula, echinecea, elderberry, garlic or vitamin C
• Drinking ginger tea several times a day
• Taking supportive homeopathic remedies (Aconite and Rhus Tox are common chickenpox remedies, but there are many others depending on the patient and the stage of illness)
• Using diluted neem or diluted essential oils such as tea tree or lavender
• Consuming plentiful bone broth to reduce digestive demands and support healing
Seek medical attention if any of the following symptoms develop: the chickenpox rash becomes tender, warm or very red; the rash has spread to one or both eyes; or there is vomiting, shortness of breath, dizziness, stiff neck, involuntary muscle movements or fever over 102 degrees Fahrenheit.
NOTE: Never use aspirin or aspirin-containing products to relieve symptoms of chickenpox. In children with chickenpox (or influenza), aspirin is associated with Reye syndrome, a severe and potentially fatal disease that affects the blood, brain and liver.
REFERENCES
- Centers for Disease Control and Prevention. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1996;45(No. RR-11).
- Meyer PA, Seward JF, Jumaan AO, Wharton M. Varicella mortality: trends before vaccine licensure in the United States, 1970-1994. J Infect Dis 2000;182(2):383-90.
- Roush SW, Murphy TV, Vaccine-Preventable Disease Table Working Group. Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA 2007;298(18):2155-63.
- “Varicella vaccine mandates for child care and K-12.” https://www.immunize.org/laws/varicella.asp.
- “Chickenpox (varicella) vaccines.” https://www.cdc.gov/vaccinesafety/vaccines/varicella-vaccine.html.
- Harvard Pilgrim Health Care, Inc. Electronic Support for Public Health—Vaccine Adverse Event Reporting System (ESP:VAERS). Rockville, MD: The Agency for Healthcare Research and Quality (AHRQ); 2011.
- “Vaccine excipient summary: excipients included in U.S. vaccines, by vaccine.” https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/b/excipient-table-2.pdf.
- Sakaguchi M, Nakayama T, Fujita H et al. Minimum estimated incidence in Japan of anaphylaxis to live virus vaccines including gelatin. Vaccine 2000;19(4-5):431-6.
- Samsel A, Seneff S. Glyphosate pathways to modern diseases VI: prions, amyloidoses and autoimmune neurological diseases. J Biol Phys Chem 2017;17:8-32.
- Zeratsky K. What is MSG? Is it bad for you? https://www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/expert-answers/monosodium-glutamate/faq-20058196.
- Choi DW. Excitotoxic cell death . J Neurobiol 1992;23(9):1261-76.
- Restani P, Ballabio C, Cattaneo A et al. Characterization of bovine serum albumin epitopes and their role in allergic reactions. Allergy 2004;59 Suppl 78:21-4.
- Debiec H, Lefeu F, Kemper MJ et al. Early childhood membranous nephropathy due to cationic bovine serum albumin. N Engl J Med 2011; 364:2101-10.
- Cotterman M. Unintended consequences: dirty genes in vaccines. The Vaccine Reaction, May 15, 2019.
- Ratajczak HV. Theoretical aspects of autism: causes—a review. J Immunotoxicol 2011;8(1):68-79.
- Pesonen E, Andsberg E, Ohlin H et al. Dual role of infections as risk factors for coronary heart disease. Atherosclerosis 2007;192(2):370-5.
- Amirian ES, Scheurer ME, Zhou R et al. History of chickenpox in glioma risk: a report from the Glioma International Case-Control Study (GICC). Cancer Med 2016;5(6):1352-8.
- Goldman GS. Universal varicella vaccination: efficacy trends and effect on herpes zoster. Int J Toxicol 2005;24(4):205-13.
- Duncan JR, Witkop CT, Webber BJ, Costello AA. Varicella seroepidemiology in United States air force recruits: a retrospective cohort study comparing immunogenicity of varicella vaccination and natural infection. Vaccine 2017;35(18):2351-7.
- Wise RP, Salive ME, Braun MM et al. Postlicensure safety surveillance for varicella vaccine. JAMA 2000;284(10):1271-9.
- “What is the history of chickenpox in America and other countries?” https://www.nvic.org/vaccines-and-diseases/chickenpox/history.aspx.
- Anderson EJ. Rotavirus vaccines: viral shedding and risk of transmission. Lancet 2008;8(10):642-9.
- ProQuad package insert. https://www.fda.gov/media/75203/download.
- Raines K, Fisher BL. CDC accused of manipulating shingles data. The Vaccine Reaction, August 19, 2018.
- “Shingles (herpes zoster): about shingles.” https://www.cdc.gov/shingles/about/index.html.
- “Shingles (herpes zoster): burden and trends.” https://www.cdc.gov/shingles/surveillance.html.
- Civen R, Chaves S, Jumaan A et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J 2009;28(11):954-9.
- Guffey DJ, Koch SB, Bomar L, Huang WW. Herpes zoster following varicella vaccination in children. Cutis 2017;99(3):207-11.
- Chun C, Weinmann S, Riedlinger K et al. Laboratory characteristics of suspected herpes zoster in vaccinated children. Pediatr Infect Dis J 2011;30(8):719-21.
- Yih WK, Brooks DR, Lett SM et al. The incidence of varicella and herpes zoster in Massachusetts as measured by the Behavioral Risk Factor Surveillance System (BRFSS) during a period of increasing varicella vaccine coverage, 1998-2003. BMC Public Health 2005;5:68.
- Mercola J. Is the chickenpox vaccine creating a shingles epidemic? The Vaccine Reaction, December 20, 2018.
- Raines K. Merck sued over Zostavax-related injury. The Vaccine Reaction, September 20, 2018.
- Lai YC, Yew YW. Severe autoimmune adverse events post herpes zoster vaccine: a case-control study of adverse events in a national database. J Drugs Dermatol 2015;14(7);681-4.
- Fried RE. Herpes zoster. N Engl J Med 2013;369;1765-6.
- “Shingles vaccine lawsuit & settlement payouts.” https://www.consumerprotect.com/defective-drugs/shingles-vaccine-lawsuit-settlement-payouts/.
- “How effective is shingles vaccine?” https://www.nvic.org/vaccines-and-diseases/shingles/vaccine-effectiveness.aspx#_edn3.
- Zostavax package insert. https://www.fda.gov/media/75975/download.
- US Claims. Merck to face growing number of Zostavax lawsuit claims. July 12, 2019. https://www.usclaims.com/Merck-to-Face-Growing-Number-of-Zostavax-Lawsuits.
- Skootsky SA. Live attenuated varicella-zoster vaccine: is it worth it? UCLA HealthCare 2006;10. http://www.med.ucla.edu/modules/xfsection/print.php?articleid=294.
- Esposito L. A new shingles vaccine: prepare for harsher side effects. U.S. News & World Report, June 15, 2018.
- Didierlaurent AM, Laupèze B, Di Pasquale A et al. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines 2017;16(1):55-63.
- Marty-Roix R, Vladimer GI, Pouliot K et al. Identification of QS-21 as an inflammasome-activating molecular component of saponin adjuvants. J Biol Chem 2016;291(3):1123-36.
- Zhu D, Tuo W. QS-21: a potent vaccine adjuvant. Nat Prod Chem Res 2016;3(4):e113.
- “U.S. births are at record-low levels—why aren’t we asking why?” Children’s Health Defense, July 31, 2019.
- Sagonowsky E. GlaxoSmithKline’s Shingrix supply will vault upward, CEO says—in 2024, that is. FiercePharma, November 15, 2019.
- Raines K. New supercharged shingles vaccine has serious problems. The Vaccine Reaction, September 4, 2018.
- Shingrix package insert. https://ca.gsk.com/media/1350785/patient-information-english.pdf.
- “Can shingles vaccine cause injury and death?” https://www.nvic.org/vaccines-and-diseases/shingles/vaccine-injury.aspx.
- Perro M, Adams V. What’s Making Our Children Sick? How Industrial Food Is Causing an Epidemic of Chronic Illness, and What Parents (and Doctors) Can Do About It. White River Junction, VT: Chelsea Green Publishing; 2017.
- “Developmental Disabilities: Facts.” Centers for Disease Control and Prevention. https://www.cdc.gov/ncbddd/developmentaldisabilities/facts.html.
- “Most recent national asthma data.” Centers for Disease Control and Prevention. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm.
- Zablotsky B, Black LI, Blumberg SJ. Estimated prevalence of children with diagnosed developmental disabilities in the United States, 2014-2016. National Center for Health Statistics Data Brief, No. 291, November 2017.
This article appeared in Wise Traditions in Food, Farming and the Healing Arts, the quarterly journal of the Weston A. Price Foundation, Spring 2020
🖨️ Print post

I just have some questions.
This goes against the “terrain” theory, which Sally Fallon and Dr. Cowan and others subscribe to.
Do you have an opinion on this?
I think your question is very valid — I’ve been wondering that too. Thanks for asking the author.
Nothing against this author, the article is very well written. But the entire virus theory on which this article is based totally goes against everything Sally and Tom Cowan wrote about in The Contagion Myth and other things WAPF has been saying since March 2020.
It’s nonsense. All my friends, who had chicken pox vaccinations got it. I however didn’t get it, until I was 21 when I got it from my housemates children. At 21 it almost killed me. It was much worse. The kids chicken pox came and went, very mildly, after 2 weeks it was gone. As an adult, it lasted 6 weeks, with compounding of bubbles. Get exposed to the chicken pox as early as you can, don’t listen to the hype on vaccines, it’s all marketing and “expert” misdirection and deception. And most of the vaccines seem to attack women and mens reproduction organs. And that’s deliberate.
Yes, saying chickenpox is caused by a virus contradicts The Contagion Myth. My understanding is that zillions of viruses are ‘in us’ – they are not ‘out there’ waiting for us to stroll by so that they can pounce and infect us. This widely accepted idea of viruses is profoundly stupid! My understanding is that when a virus that is already in us is triggered by something ‘out there,’ the something being a toxin, such as poisons in our food, water, and air pollution, especially EMF and 5G rollout, if our immune system is overwhelmed by the toxin, certain of our viruses (exosomes) will become pathogenic, expressing themselves in one disease or another.
Chickenpox, therefore, is not caused by a virus. To rid the world of useless and disease causing vaccines, it will be necessary to rid the world of its belief in Pasteur’s GERM THEORY.
The phrase in my comment “certain of our viruses will become pathogenic” is incorrect. What is actually happening is that the toxin is affecting the cells, directly causing the cells to become pathogenic. Therefore, the viruses (exosomes) in our body do not become pathogenic and cause disease.
When reading articles about Covid, it helps immensely if I keep in mind the fact that the supposed coronavirus does not exist. The proof of the non-existent coronavirus is detailed by Drs Kaufman and Cowan plus Sally Fallon Morrell here: https://www.andrewkaufmanmd.com/sovi/
What do we do for shingles?
Yes please, seconding this. Am also keen to know of any Weston Price protocol/approach for shingles.
Hi, I would also like to know the best natural remedy to reduce the pain and get over the illness